The weekly litigation news digest is live. Subscribe now
Safe Desmopressin Administration - EP2381923
The patent EP2381923 was granted to Acerus Pharmaceuticals USA on Jun 7, 2023. The application was filed on Dec 21, 2009 under application number EP09796558A. The patent is currently recorded with a legal status of "Patent Maintained As Amended".
EP2381923
- Application Number
- EP09796558A
- Filing Date
- Dec 21, 2009
- Status
- Patent Maintained As Amended
- May 5, 2023
- Publication Date
- Jun 7, 2023
- External Links
- Slate, Register, Google Patents
Patent Summary
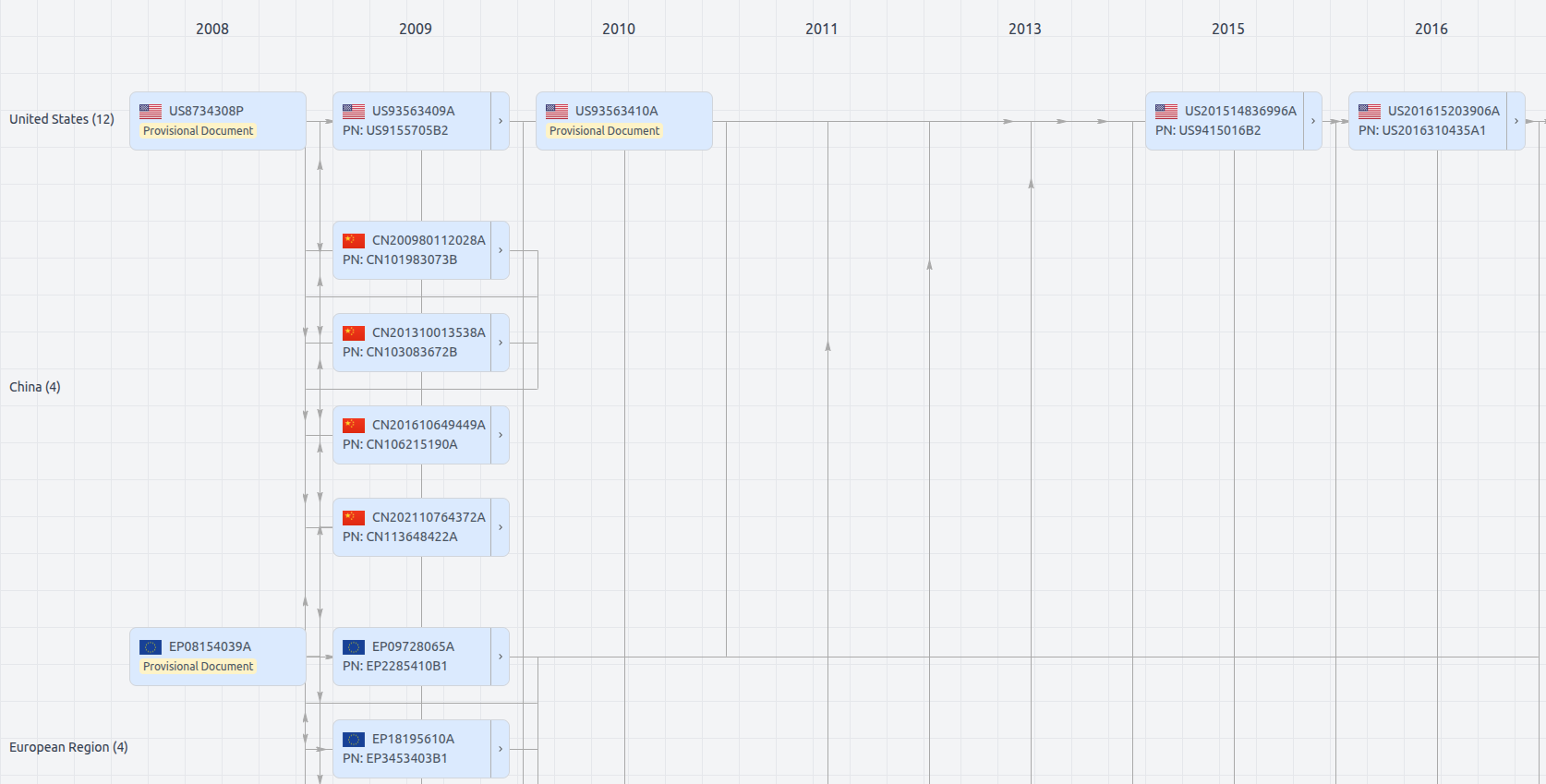
Patent Family
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Patent Citations (35) New
Patent citations refer to prior patents cited during different phases such as opposition or international search.
| Citation Phase | Publication Number |
|---|---|
| DESCRIPTION | US2003232078 |
| DESCRIPTION | US3980631 |
| DESCRIPTION | US4148787 |
| DESCRIPTION | US4548922 |
| DESCRIPTION | US4746508 |
| DESCRIPTION | US4860738 |
| DESCRIPTION | US4944429 |
| DESCRIPTION | US5023252 |
| DESCRIPTION | US5112804 |
| DESCRIPTION | US5122383 |
| DESCRIPTION | US5154122 |
| DESCRIPTION | US5212199 |
| DESCRIPTION | US5227169 |
| DESCRIPTION | US5314694 |
| DESCRIPTION | US5719122 |
| DESCRIPTION | US5731303 |
| DESCRIPTION | US6321942 |
| DESCRIPTION | US6446839 |
| DESCRIPTION | US6705493 |
| DESCRIPTION | US6708846 |
| DESCRIPTION | US6772915 |
| DESCRIPTION | US7112561 |
| DESCRIPTION | US7182226 |
| DESCRIPTION | US7244703 |
| DESCRIPTION | US7335186 |
| DESCRIPTION | US7405203 |
| INTERNATIONAL-SEARCH-REPORT | US2005158247 |
| INTERNATIONAL-SEARCH-REPORT | WO9501183 |
| OPPOSITION | US7112561 |
| OPPOSITION | US7182226 |
| OPPOSITION | US7244703 |
| OPPOSITION | US7405203 |
| OPPOSITION | US9539302 |
| OPPOSITION | WO2010075266 |
| OPPOSITION | WO9501183 |
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
May 11, 2023
May 5, 2023
Apr 27, 2023
Apr 27, 2023
Apr 27, 2023
Apr 27, 2023
Feb 17, 2023
Feb 17, 2023
Feb 14, 2023
Jan 26, 2023
Jan 23, 2023
Refund of fees
Appeal
Jan 19, 2023
Jan 16, 2023
Jan 9, 2023
(Electronic) Receipt
Appeal
Jan 9, 2023
Jan 9, 2023
Dec 1, 2022
Nov 28, 2022
(Electronic) Receipt
Appeal
Nov 28, 2022
Nov 28, 2022
Oct 10, 2022
Oct 10, 2022
Oct 4, 2022
(Electronic) Receipt
Appeal
Oct 4, 2022
Oct 4, 2022
Oct 4, 2022
Oct 4, 2022
Sep 23, 2022
(Electronic) Receipt
Appeal
Sep 23, 2022
Sep 23, 2022
Sep 23, 2022
Aug 17, 2022
Aug 11, 2022
(Electronic) Receipt
Appeal
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Aug 11, 2022
Reply to appeal
Appeal
May 17, 2022
May 16, 2022
May 3, 2022
May 3, 2022
Apr 11, 2022
Apr 4, 2022
(Electronic) Receipt
Appeal
Apr 4, 2022
Apr 4, 2022
Feb 8, 2022
Feb 8, 2022
Feb 8, 2022
Feb 1, 2022
(Electronic) Receipt
Appeal
Feb 1, 2022
Feb 1, 2022
Notice of appeal
Appeal
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Means of redress
OPPO
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Nov 25, 2021
Jul 1, 2021
Jun 23, 2021
Jun 23, 2021
Jun 21, 2021
Jun 21, 2021
Jun 21, 2021
Jun 15, 2021
Jun 15, 2021
Jun 11, 2021
Jun 11, 2021
Jun 11, 2021
Jun 11, 2021
Jun 11, 2021
Jun 9, 2021
Jun 9, 2021
May 12, 2021
May 12, 2021
May 7, 2021
May 7, 2021
Apr 30, 2021
Apr 30, 2021
Apr 30, 2021
Apr 30, 2021
Apr 30, 2021
Apr 30, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Apr 29, 2021
Mar 11, 2021
Mar 11, 2021
Sep 21, 2020
Sep 15, 2020
Sep 14, 2020
Sep 14, 2020
Sep 14, 2020
Sep 14, 2020
Sep 14, 2020
Sep 14, 2020
Sep 2, 2020
Sep 2, 2020
Sep 2, 2020
Sep 2, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Aug 19, 2020
Jul 15, 2020
Jul 15, 2020
Feb 3, 2020
Jan 31, 2020
Jan 24, 2020
Jan 24, 2020
Jan 24, 2020
Jan 24, 2020
Jan 24, 2020
Jan 24, 2020
Jan 21, 2020
Dec 11, 2019
Dec 5, 2019
Dec 5, 2019
Dec 5, 2019
Dec 5, 2019
Dec 5, 2019
Dec 5, 2019
Dec 5, 2019
Oct 8, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Sep 26, 2019
Jul 18, 2019
Jul 12, 2019
Jun 21, 2019
Jun 21, 2019
Jun 21, 2019
Jun 17, 2019
Jun 17, 2019
Jun 17, 2019
May 16, 2019
May 16, 2019
May 10, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
May 1, 2019
Aug 13, 2018
Jul 5, 2018
Decision to grant a European patent
Search/Exam
Jun 21, 2018
Jun 21, 2018
Jun 21, 2018
Intention to grant (signatures)
Search/Exam
Jun 21, 2018
Text intended for grant (clean copy)
Search/Exam
Jun 21, 2018
Jun 15, 2018
Jun 13, 2018
May 25, 2018
(Electronic) Receipt
Search/Exam
May 25, 2018
Amended description with annotations
Search/Exam
May 25, 2018
Description
Search/Exam
May 25, 2018
French translation of claims
Search/Exam
May 25, 2018
German translation of the claims
Search/Exam
May 25, 2018
Letter accompanying subsequently filed items
Search/Exam
May 25, 2018
Jan 16, 2018
Jan 16, 2018
Jan 16, 2018
Intention to grant (signatures)
Search/Exam
Jan 16, 2018
Text intended for grant (clean copy)
Search/Exam
Jan 16, 2018
Dec 6, 2017
Nov 27, 2017
(Electronic) Receipt
Search/Exam
Nov 27, 2017
Annexes in respect of a client data request
Search/Exam
Nov 27, 2017
Letter accompanying subsequently filed items
Search/Exam
Nov 27, 2017
Sep 29, 2017
Decision to allow further processing
Search/Exam
Sep 15, 2017
(Electronic) Receipt
Search/Exam
Sep 15, 2017
Amended claims with annotations
Search/Exam
Sep 15, 2017
Claims
Search/Exam
Sep 15, 2017
Letter accompanying subsequently filed items
Search/Exam
Sep 15, 2017
Request for further processing
Search/Exam
Jul 7, 2017
Jun 20, 2017
Jun 8, 2017
(Electronic) Receipt
Search/Exam
Jun 8, 2017
Letter accompanying subsequently filed items
Search/Exam
Jun 8, 2017
Mar 28, 2017
Mar 22, 2017
(Electronic) Receipt
Search/Exam
Mar 22, 2017
Letter accompanying subsequently filed items
Search/Exam
Mar 22, 2017
Nov 28, 2016
Annex to the communication
Search/Exam
Nov 28, 2016
Communication from the Examining Division
Search/Exam
Oct 29, 2015
(Electronic) Receipt
Search/Exam
Oct 29, 2015
Amended claims with annotations
Search/Exam
Oct 29, 2015
Claims
Search/Exam
Oct 29, 2015
Letter accompanying subsequently filed items
Search/Exam
Oct 29, 2015
Sep 2, 2015
Aug 28, 2015
(Electronic) Receipt
Search/Exam
Aug 28, 2015
Letter accompanying subsequently filed items
Search/Exam
Aug 28, 2015
May 19, 2015
Annex to the communication
Search/Exam
May 19, 2015
Communication from the Examining Division
Search/Exam
May 7, 2015
Examination started
Search/Exam
Jul 2, 2014
CDS Clean up - amended applicant details
Search/Exam
Oct 6, 2011
Sep 22, 2011
Jul 22, 2011
(Electronic) Receipt
Search/Exam
Jul 22, 2011
Letter accompanying subsequently filed items
Search/Exam
Jul 22, 2011
Matter concerning the application
Search/Exam
Jul 21, 2011
(Electronic) Receipt
Search/Exam
Jul 21, 2011
(Electronic) Receipt
Search/Exam
Jul 21, 2011
Jul 21, 2011
Amendments received before examination
Search/Exam
Jul 21, 2011
Annex
Search/Exam
Jul 21, 2011
Annex
Search/Exam
Jul 21, 2011
Annex
Search/Exam
Jul 21, 2011
Letter accompanying subsequently filed items
Search/Exam
Jul 21, 2011
Jul 21, 2011
Request for assignment
Search/Exam
Jul 21, 2011
Request for entry into the European phase
Search/Exam
Jul 21, 2011
Submission concerning representation
Search/Exam
Jul 13, 2011
Jul 11, 2011
Notification of the recording of a change
Search/Exam
Jul 11, 2011
Notification of the recording of a change
Search/Exam
May 9, 2011
Information on entry into European phase
Search/Exam
Jan 6, 2011
Copy of the international search report
Search/Exam
Jan 6, 2011
Nov 17, 2010
Nov 17, 2010
Nov 17, 2010
Jul 23, 2010
Jul 1, 2010
Published international application - A2
Search/Exam
Jan 11, 2010
Abstract
PCTISA
Jan 11, 2010
Claims
PCTISA
Jan 11, 2010
Description
PCTISA
Jan 11, 2010
Drawings
PCTISA