The weekly litigation news digest is live. Subscribe now
Sacubitril-Valsartan Dosage Regimen For Treating Heart Failure - EP3294283
The patent EP3294283 was granted to Novartis on Mar 8, 2023. The application was filed on May 9, 2016 under application number EP16722388A. The patent is currently recorded with a legal status of "Granted And Under Opposition".
EP3294283
- Application Number
- EP16722388A
- Filing Date
- May 9, 2016
- Status
- Granted And Under Opposition
- Feb 3, 2023
- Publication Date
- Mar 8, 2023
- External Links
- Slate, Register, Google Patents
Patent Summary
Treatment of heart failure in a human patient that prevents the onset of a disease characterized and / or manifested by atrial enlargement and/or remodeling. The treatment comprises administering to a patient in need thereof a twice-daily target dose of an Angiotensin Receptor Neprilysin inhibitor (ARNi) or of a combination of an Angiotensin Receptor Blocker (ARB) with a Neutral Endopeptidase inhibitor (NEPi) or with a NEPi pro-drug, wherein said target dose is reached after a titration with an initial lower twice-daily dose of said ARNI increasing to the twice daily target dose from 5 to 8 weeks.
Patent Family
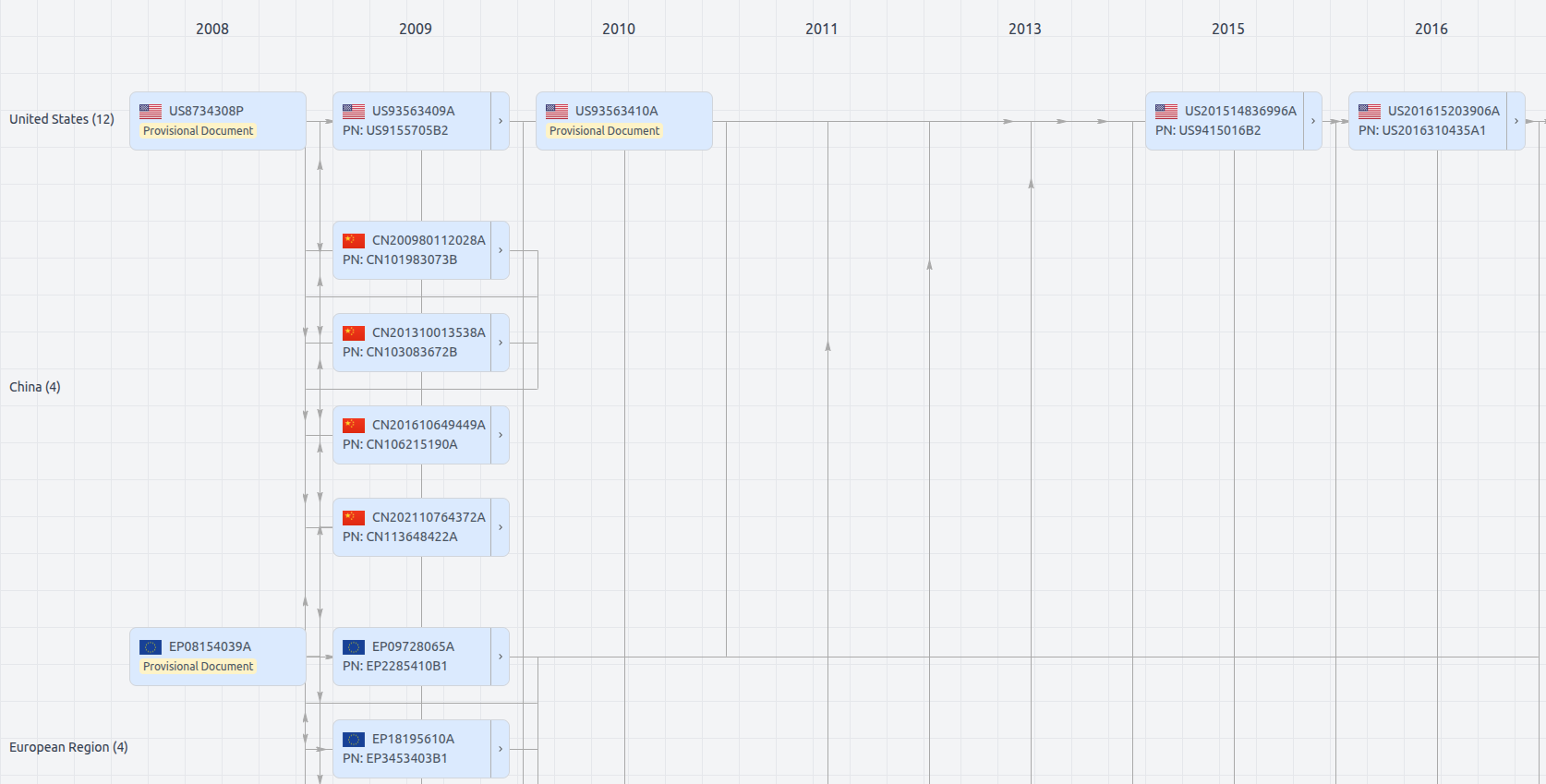
Patent Oppositions (16)
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
| Company | Opposition Date | Representative | Opposition Status |
|---|---|---|---|
| BULLE DR | Dec 8, 2023 | KUTZENBERGER WOLFF & PARTNER | ADMISSIBLE |
| KRKA | Dec 8, 2023 | HOFFMANN EITLE | ADMISSIBLE |
| ZENTIVA | Dec 8, 2023 | GREINER | ADMISSIBLE |
| ALFRED E TIEFENBACHER | Dec 7, 2023 | HAMM & WITTKOPP | ADMISSIBLE |
| BETAPHARM ARZNEIMITTEL | Dec 7, 2023 | HAMM & WITTKOPP | ADMISSIBLE |
| GENERICS UK | Dec 7, 2023 | ELKINGTON AND FIFE | ADMISSIBLE |
| POLPHARMA | Dec 7, 2023 | HAMM & WITTKOPP | ADMISSIBLE |
| SANOVEL IIAC SAN VE TIC | Dec 7, 2023 | KRAUS & LEDERER | ADMISSIBLE |
| SANOVEL IIAC SAN VE TIC | Dec 7, 2023 | KRAUS & LEDERER PARTGMBB | ADMISSIBLE |
| STRAWMAN | Dec 7, 2023 | PINSENT MASONS | ADMISSIBLE |
| SYNTHON | Dec 7, 2023 | HAMM & WITTKOPP | ADMISSIBLE |
| TEVA PHARMACEUTICALS | Dec 7, 2023 | D YOUNG | ADMISSIBLE |
| HOFFMANN EITLE | Dec 4, 2023 | HOFFMANN EITLE | WITHDRAWN |
| DR SCHON | Nov 30, 2023 | DR SCHON | ADMISSIBLE |
| CAMULON | Nov 23, 2023 | REDDIE & GROSE | ADMISSIBLE |
| EGIS | Oct 9, 2023 | STOLMAR & PARTNER | ADMISSIBLE |
Patent Citations (11) New
Patent citations refer to prior patents cited during different phases such as opposition or international search.
| Citation Phase | Publication Number |
|---|---|
| DESCRIPTION | EP0443983 |
| DESCRIPTION | US5217996 |
| DESCRIPTION | US5399578 |
| DESCRIPTION | WO03059345 |
| DESCRIPTION | WO2007056546 |
| DESCRIPTION | WO2009061713 |
| DESCRIPTION | WO2014029848 |
| OPPOSITION | WO03059345 |
| OPPOSITION | WO2014029848 |
| OPPOSITION | WO2015030711 |
| OPPOSITION | WO2016181284 |
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
Jan 13, 2026
(Electronic) Receipt
Appeal
Jan 13, 2026
Jan 13, 2026
Notice of appeal
Appeal
Jan 12, 2026
Jan 12, 2026
Jan 12, 2026
Jan 12, 2026
Jan 9, 2026
Jan 7, 2026
(Electronic) Receipt
Appeal
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Jan 7, 2026
Notice of appeal
Appeal
Jan 5, 2026
(Electronic) Receipt
Appeal
Jan 5, 2026
Notice of appeal
Appeal
Dec 23, 2025
Dec 23, 2025
Dec 22, 2025
Dec 22, 2025
Dec 22, 2025
Dec 17, 2025
(Electronic) Receipt
Appeal
Dec 17, 2025
(Electronic) Receipt
Appeal
Dec 17, 2025
Notice of appeal
Appeal
Dec 17, 2025
Notice of appeal
Appeal
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Means of redress
OPPO
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Dec 12, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 10, 2025
Nov 10, 2025
Nov 10, 2025
Nov 7, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 24, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 21, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 10, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Oct 2, 2025
Sep 12, 2025
Sep 12, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 10, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 9, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 8, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 5, 2025
Sep 4, 2025
Sep 4, 2025
Sep 4, 2025
Sep 4, 2025
Sep 4, 2025
Sep 4, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 3, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 2, 2025
Sep 1, 2025
Sep 1, 2025
Sep 1, 2025
Sep 1, 2025
Sep 1, 2025
Sep 1, 2025
Aug 29, 2025
Aug 29, 2025
Aug 29, 2025
Aug 29, 2025
Aug 29, 2025
Aug 29, 2025
Aug 29, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Aug 27, 2025
Feb 7, 2025
Feb 7, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 15, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 13, 2025
Jan 9, 2025
Jan 9, 2025
Jan 9, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Nov 27, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 23, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 9, 2024
Oct 9, 2024
Oct 9, 2024
Oct 9, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Oct 7, 2024
Jul 24, 2024
Jul 24, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 26, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Jun 20, 2024
Mar 13, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 22, 2024
Jan 11, 2024
Jan 11, 2024
Jan 11, 2024
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 20, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 14, 2023
Dec 12, 2023
Letter accompanying subsequently filed items
Search/Exam
Dec 12, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 8, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 7, 2023
Dec 6, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Dec 4, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 30, 2023
Nov 29, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 23, 2023
Nov 22, 2023
Oct 20, 2023
Oct 19, 2023
Oct 19, 2023
Oct 19, 2023
Oct 16, 2023
Oct 16, 2023
Oct 13, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Aug 4, 2023
Jul 18, 2023
(Electronic) Receipt
Search/Exam
Jul 18, 2023
Letter accompanying subsequently filed items
Search/Exam
Jul 18, 2023
Jul 18, 2023
Mar 21, 2023
Feb 15, 2023
Feb 9, 2023
Decision to grant a European patent
Search/Exam
Feb 9, 2023
Non-patent literature filed by a third party
Search/Exam
Feb 9, 2023
Non-patent literature filed by a third party
Search/Exam
Feb 9, 2023
Non-patent literature filed by a third party
Search/Exam
Feb 9, 2023
Non-patent literature filed by a third party
Search/Exam
Feb 9, 2023
Observations by third parties
Search/Exam
Feb 9, 2023
Observations by third parties
Search/Exam
Jan 31, 2023
Jan 30, 2023
Jan 30, 2023
Jan 25, 2023
(Electronic) Receipt
Search/Exam
Jan 25, 2023
Letter accompanying subsequently filed items
Search/Exam
Jan 25, 2023
Non-patent literature filed by a third party
Search/Exam
Jan 25, 2023
Non-patent literature filed by a third party
Search/Exam
Jan 25, 2023
Observations by third parties
Search/Exam
Jan 23, 2023
(Electronic) Receipt
Search/Exam
Jan 23, 2023
French translation of claims
Search/Exam
Jan 23, 2023
German translation of the claims
Search/Exam
Jan 23, 2023
Letter accompanying subsequently filed items
Search/Exam
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Intention to grant (signatures)
Search/Exam
Dec 7, 2022
Text intended for grant (clean copy)
Search/Exam
Dec 7, 2022
Aug 16, 2022
(Electronic) Receipt
Search/Exam
Aug 16, 2022
Letter accompanying subsequently filed items
Search/Exam
Aug 16, 2022
Matter concerning the application
Search/Exam
Aug 16, 2022
Jul 14, 2022
Decision to allow further processing
Search/Exam
Jul 5, 2022
(Electronic) Receipt
Search/Exam
Jul 5, 2022
Amended claims with annotations
Search/Exam
Jul 5, 2022
Amended description with annotations
Search/Exam
Jul 5, 2022
Claims
Search/Exam
Jul 5, 2022
Description
Search/Exam
Jul 5, 2022
Letter accompanying subsequently filed items
Search/Exam
Jul 5, 2022
Request for further processing
Search/Exam
May 17, 2022
Feb 8, 2022
Feb 3, 2022
(Electronic) Receipt
Search/Exam
Feb 3, 2022
Letter accompanying subsequently filed items
Search/Exam
Feb 3, 2022
Dec 22, 2021
Dec 17, 2021
(Electronic) Receipt
Search/Exam
Dec 17, 2021
Letter accompanying subsequently filed items
Search/Exam
Dec 17, 2021
Oct 12, 2021
Annex to the communication
Search/Exam
Oct 12, 2021
Communication from the Examining Division
Search/Exam
Oct 6, 2021
Jan 8, 2021
(Electronic) Receipt
Search/Exam
Jan 8, 2021
Amended claims with annotations
Search/Exam
Jan 8, 2021
Claims
Search/Exam
Jan 8, 2021
Letter accompanying subsequently filed items
Search/Exam
Jan 8, 2021
Nov 3, 2020
Oct 28, 2020
(Electronic) Receipt
Search/Exam
Oct 28, 2020
Letter accompanying subsequently filed items
Search/Exam
Oct 28, 2020
Jun 18, 2020
Annex to the communication
Search/Exam
Jun 18, 2020
Communication from the Examining Division
Search/Exam
Feb 20, 2020
(Electronic) Receipt
Search/Exam
Feb 20, 2020
Amended claims with annotations
Search/Exam
Feb 20, 2020
Claims
Search/Exam
Feb 20, 2020
Letter accompanying subsequently filed items
Search/Exam
Feb 20, 2020
Dec 19, 2019
Dec 13, 2019
(Electronic) Receipt
Search/Exam
Dec 13, 2019
Letter accompanying subsequently filed items
Search/Exam
Dec 13, 2019
Aug 12, 2019
Annex to the communication
Search/Exam
Aug 12, 2019
Communication from the Examining Division
Search/Exam
Aug 6, 2019
Examination started
Search/Exam
Feb 21, 2018
Dec 22, 2017
Dec 20, 2017
Communication to designated inventor
Search/Exam
Dec 20, 2017
Communication to designated inventor
Search/Exam
Dec 20, 2017
Communication to designated inventor
Search/Exam
Nov 27, 2017
Nov 10, 2017
(Electronic) Receipt
Search/Exam
Nov 10, 2017
Nov 10, 2017
Request for entry into the European phase
Search/Exam
Sep 22, 2017
Information on entry into European phase
Search/Exam
Dec 6, 2016
Nov 17, 2016
Copy of the international search report
Search/Exam
Nov 17, 2016
Published international application - A1
Search/Exam
Jun 22, 2016
Jun 22, 2016
Jun 22, 2016