Sodium (2R, 5S, 13Ar) -7, 9-Dioxo-10- ( (2,4,6-Trifluorobenzyl) Carbamoyl) -2, 3, 4, 5, 7, 9, 13, 13A-Octahydro-2, 5-Methanopyrido [1',2' : 4.5] Pyrazino [2, 1-B] Oxazepin-8-Olate
Patent No. EP3321270 (titled "Sodium (2R, 5S, 13Ar) -7, 9-Dioxo-10- ( (2,4,6-Trifluorobenzyl) Carbamoyl) -2, 3, 4, 5, 7, 9, 13, 13A-Octahydro-2, 5-Methanopyrido [1',2' : 4.5] Pyrazino [2, 1-B] Oxazepin-8-Olate") was filed by Gilead Sciences on Jun 19, 2015. The application was issued on Dec 4, 2024.
Patent Summary
Crystalline sodium salt of a HIV protease inhibitor, specifically (2R,5S,13aR)-7,9-dioxo-10-((2,4,6-trifluorobenzyl)carbamoyl)-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepin-8-olate, in a stable Form I polymorph, suitable for pharmaceutical formulations and treatment of HIV infection.
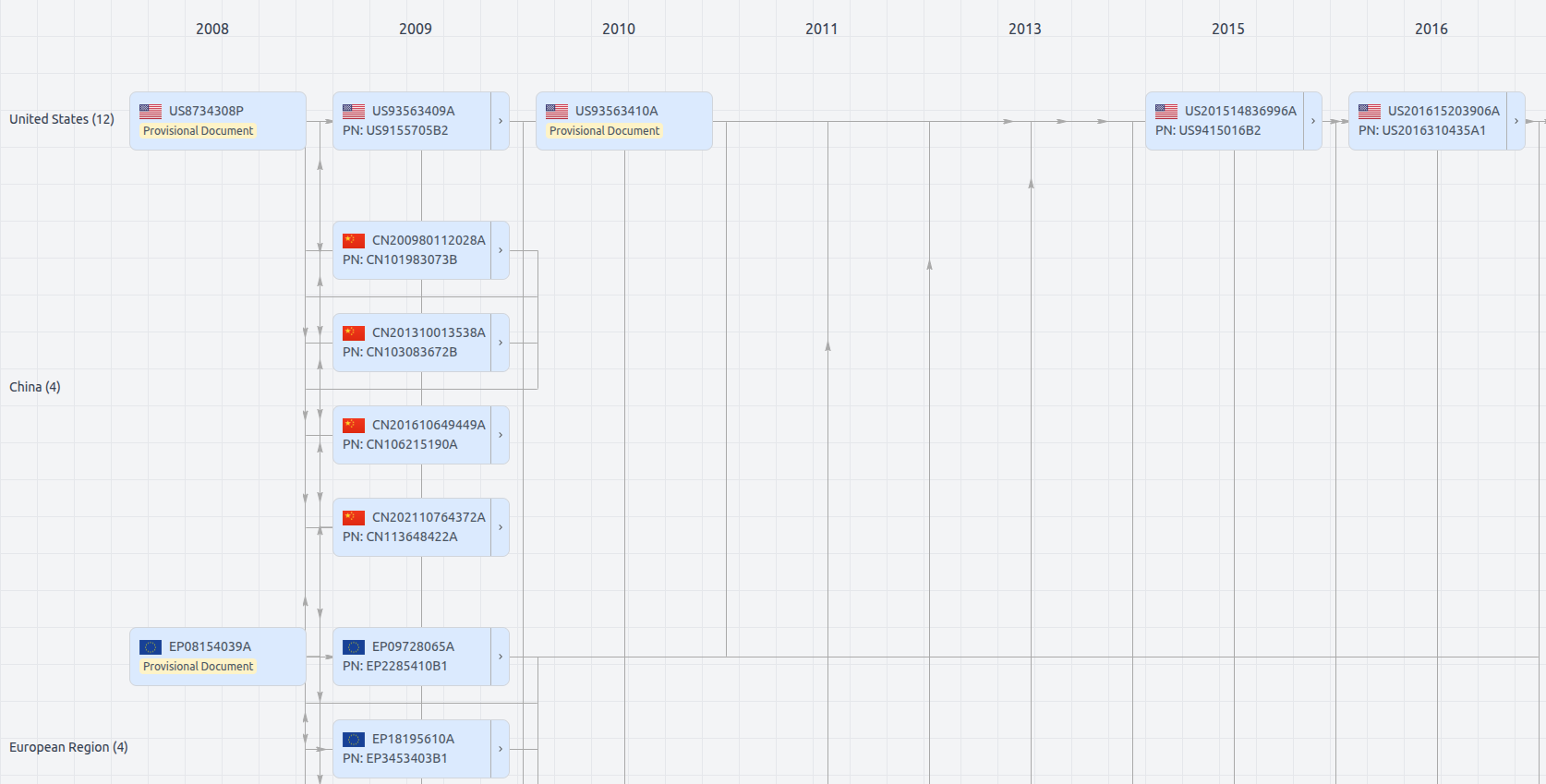
Patent Family
Patent Oppositions (5)
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP3321270
- Application Number
- EP17204116A
- Filing Date
- Jun 19, 2015
- Status
- Granted And Under Opposition
- Nov 1, 2024
- Publication Date
- Dec 4, 2024
- External Links
- Slate, Register , Google Patents