The weekly litigation news digest is live. Subscribe now
Composite Capsule Preparation Containing Tadalafil And Tamsulosin And Having Improved Stability And Elution Rate - EP3421034
The patent EP3421034 was granted to Hanmi Pharm on Nov 16, 2022. The application was filed on Mar 31, 2017 under application number EP17775932A. The patent is currently recorded with a legal status of "Revoked".
EP3421034
- Application Number
- EP17775932A
- Filing Date
- Mar 31, 2017
- Status
- Revoked
- Jan 24, 2025
- Publication Date
- Nov 16, 2022
- External Links
- Slate, Register, Google Patents
Patent Summary
Capsule composite formulation containing both tadalafil and tamsulosin for treating erectile dysfunction and benign prostatic hyperplasia simultaneously. The formulation has improved stability and dissolution of both drugs compared to separate capsules. The key is coating the tadalafil part with a film layer containing PVA. This prevents interaction between tadalafil and tamsulosin when combined in a capsule. The PVA coating on tadalafil provides stability without affecting its dissolution. The tamsulosin part can have an enteric coating. The tadalafil part is a compressed tablet of granules with a mix of larger and smaller particles.
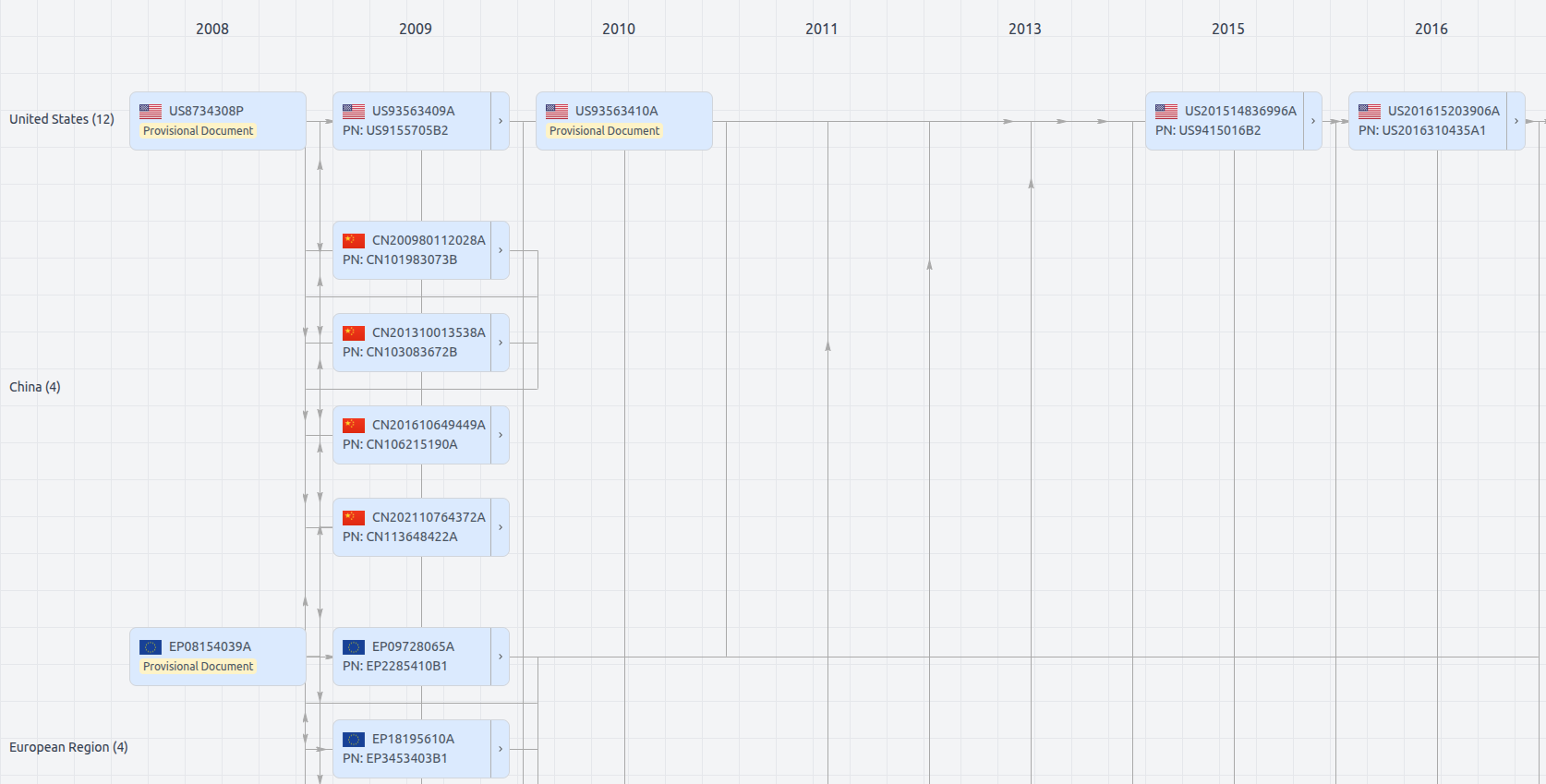
Patent Family
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Patent Citations (11) New
Patent citations refer to prior patents cited during different phases such as opposition or international search.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents