Dry Powder Inhaler And Methods Of Use
Patent No. EP3613421 (titled "Dry Powder Inhaler And Methods Of Use") was filed by Aspeya on Mar 15, 2013. The application was issued on May 3, 2023.
Patent Summary
Reducing the risk of thromboembolic events like heart attack or stroke by inhaling very low doses of aspirin using a dry powder inhaler. The doses are much lower than traditional oral aspirin, ranging from 2 mg to 10 mg. This enables faster absorption and action compared to oral aspirin due to avoiding the first pass effect in the gut. The low doses by inhalation also mitigate bleeding risks associated with higher aspirin doses.
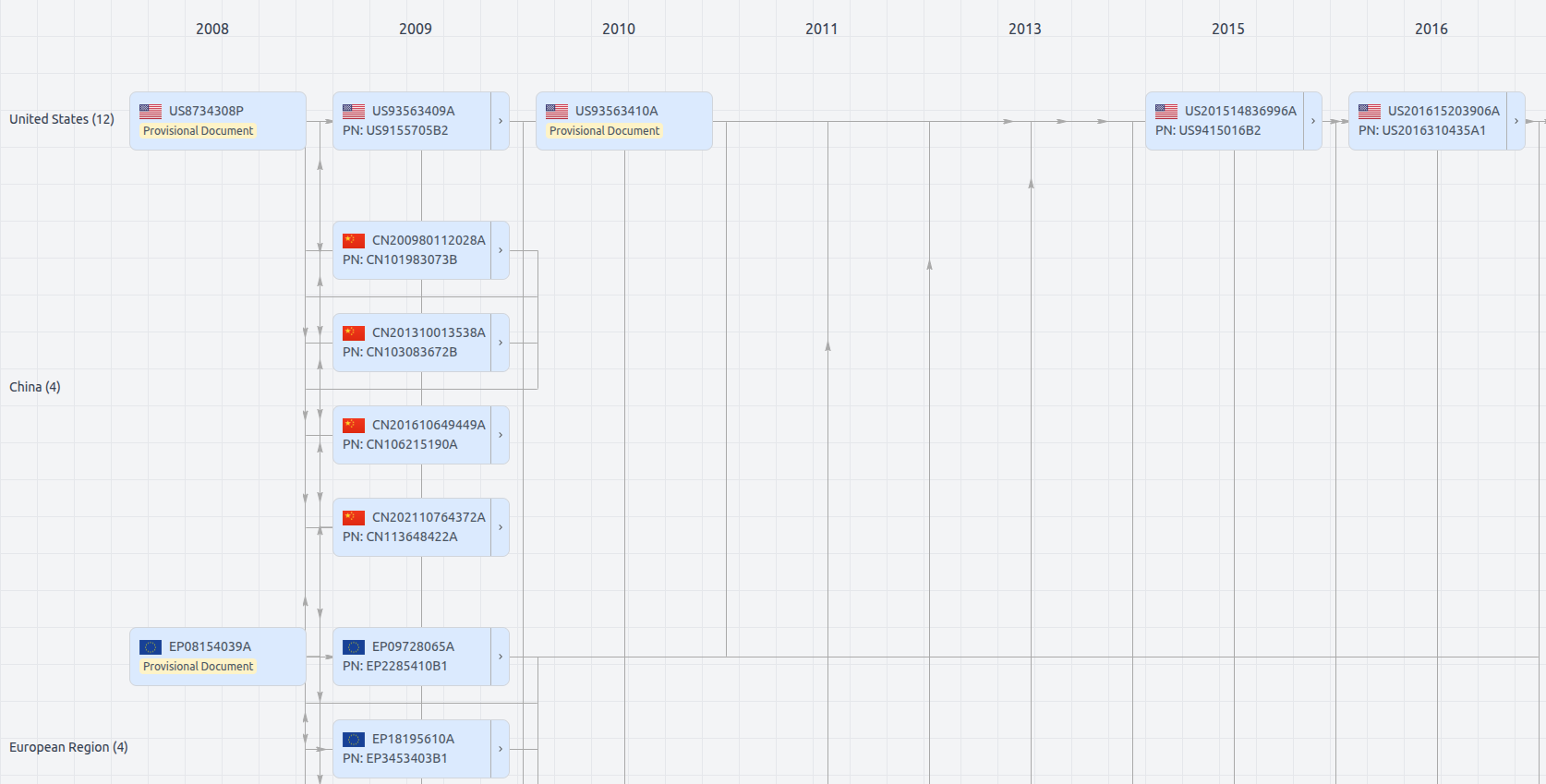
Patent Family
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP3613421
- Application Number
- EP19194560A
- Filing Date
- Mar 15, 2013
- Status
- Granted And Under Opposition
- Mar 31, 2023
- Publication Date
- May 3, 2023
- External Links
- Slate, Register , Google Patents