Pharmaceutical Composition Comprising Selexipag
Patent No. EP3592391 (titled "Pharmaceutical Composition Comprising Selexipag") was filed by Actelion Pharmaceuticals on Mar 7, 2018. The application was issued on Apr 21, 2021.
Patent Summary
Stable i.v. formulation of the drug selexipag for injection. The formulation is a lyophilized powder that can be reconstituted for injection. It contains selexipag, a bulking agent, a detergent, and a buffer. The composition has controlled selexipag concentration, pH, osmolality, and cake properties for stable lyophilization and reconstitution. This allows reliable and quantitative dosing of selexipag for injection.
Patent Family
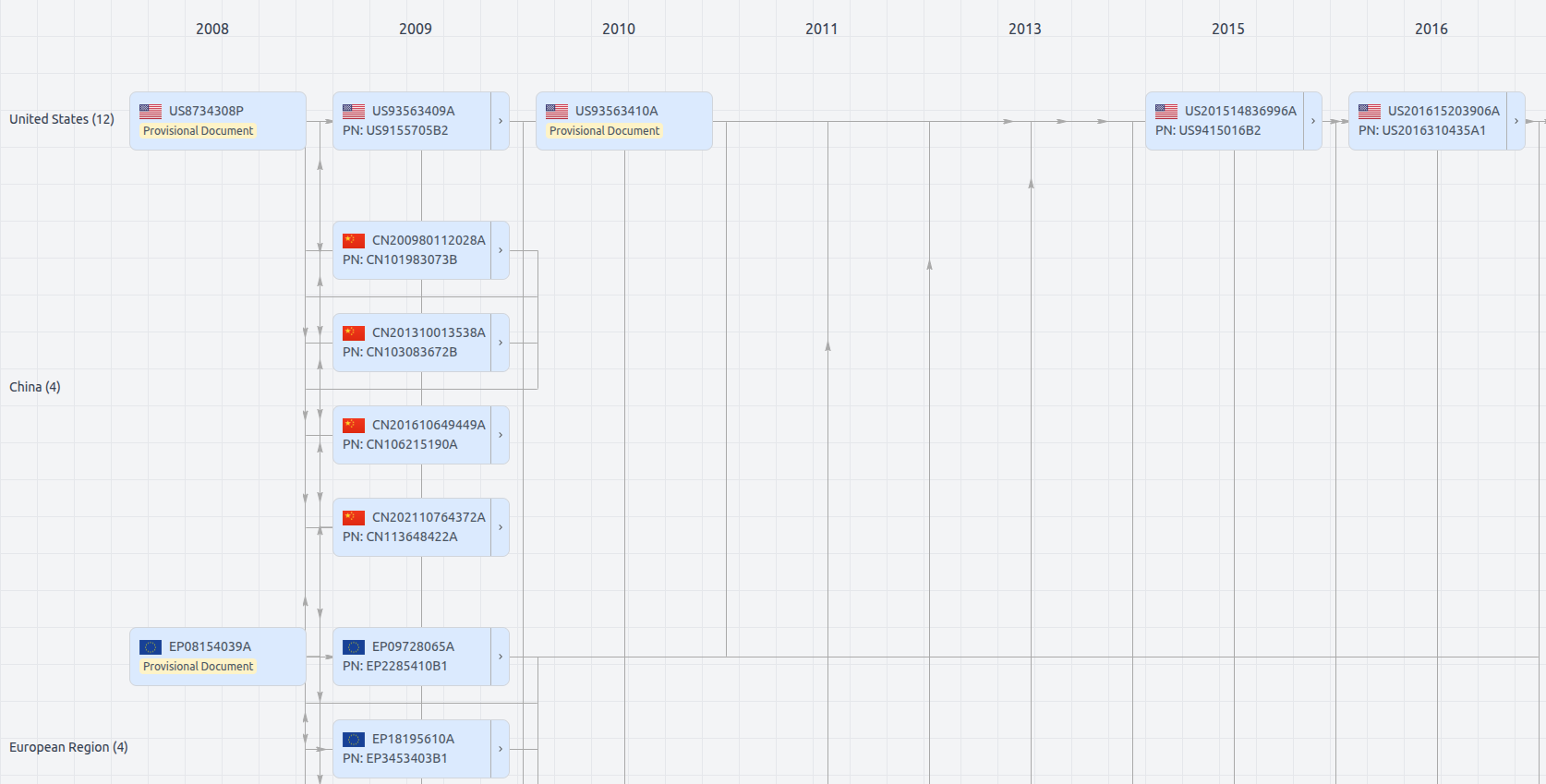
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP3592391
- Application Number
- EP18711258A
- Filing Date
- Mar 7, 2018
- Status
- Granted And Under Opposition
- Mar 19, 2021
- Publication Date
- Apr 21, 2021
- External Links
- Slate, Register , Google Patents