Edaravone Suspension For Oral Administration
Patent No. EP3875085 (titled "Edaravone Suspension For Oral Administration") was filed by Mitsubishi Tanabe Pharma on Nov 1, 2019. The application was issued on Jun 19, 2024.
Patent Summary
Edaravone suspension for oral administration that achieves therapeutic efficacy comparable to intravenous injections while reducing the burden on patients and caregivers. The suspension contains edaravone particles dispersed in a water-based solution with added stabilizers and pH regulators. The formulation enables sustained release of edaravone through the gastrointestinal tract, with a dosing regimen of 90-120 mg per oral administration.
Patent Family
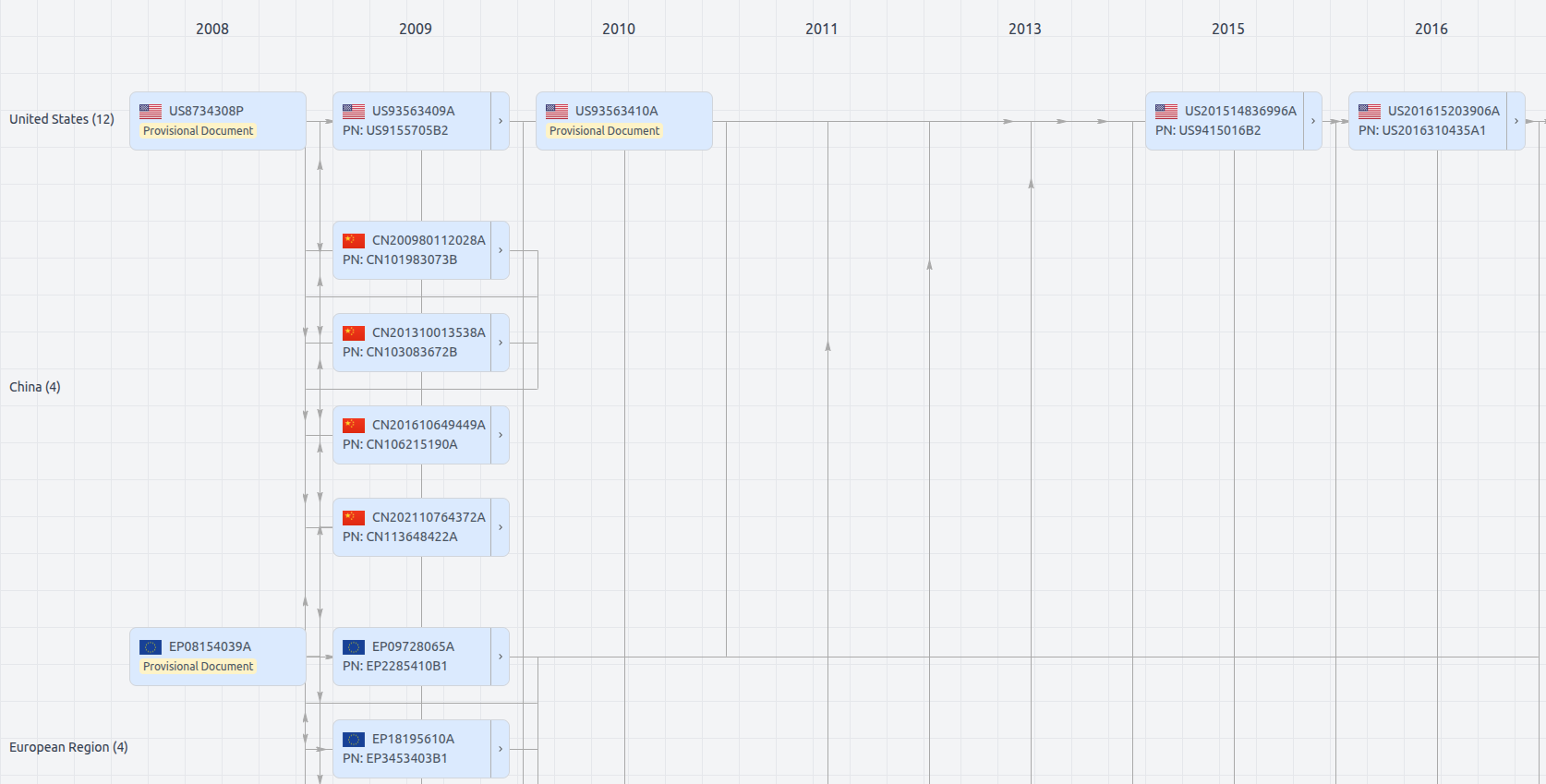
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP3875085
- Application Number
- EP19880770A
- Filing Date
- Nov 1, 2019
- Status
- Granted And Under Opposition
- May 17, 2024
- Publication Date
- Jun 19, 2024
- External Links
- Slate, Register , Google Patents