Polymorphs Of (R)-N-(5-(5-Ethyl-1,2,4-Oxadiazol-3-Yl)-2,3-Dihydro-1H-Inden-1-Yl)-1-Methyl-1H-Pyrazole-4-Carboxamide
Patent No. EP3999180 (titled "Polymorphs Of (R)-N-(5-(5-Ethyl-1,2,4-Oxadiazol-3-Yl)-2,3-Dihydro-1H-Inden-1-Yl)-1-Methyl-1H-Pyrazole-4-Carboxamide") was filed by Cytokinetics on Jul 16, 2020. The application was issued on May 8, 2024.
Patent Summary
Polymorphs of a therapeutic agent for treating cardiac diseases, particularly hypertrophic cardiomyopathy (HCM) and heart failure with preserved ejection fraction (HFpEF), that exhibit enhanced stability and therapeutic index compared to conventional forms. The polymorphs have distinct crystal structures that differ in physical properties, such as melting points, solubilities, and dissolution rates, which enable controlled manufacturing processes. These polymorphs retain the therapeutic activity of the parent compound while reducing adverse effects, making them a promising alternative for treating these conditions.
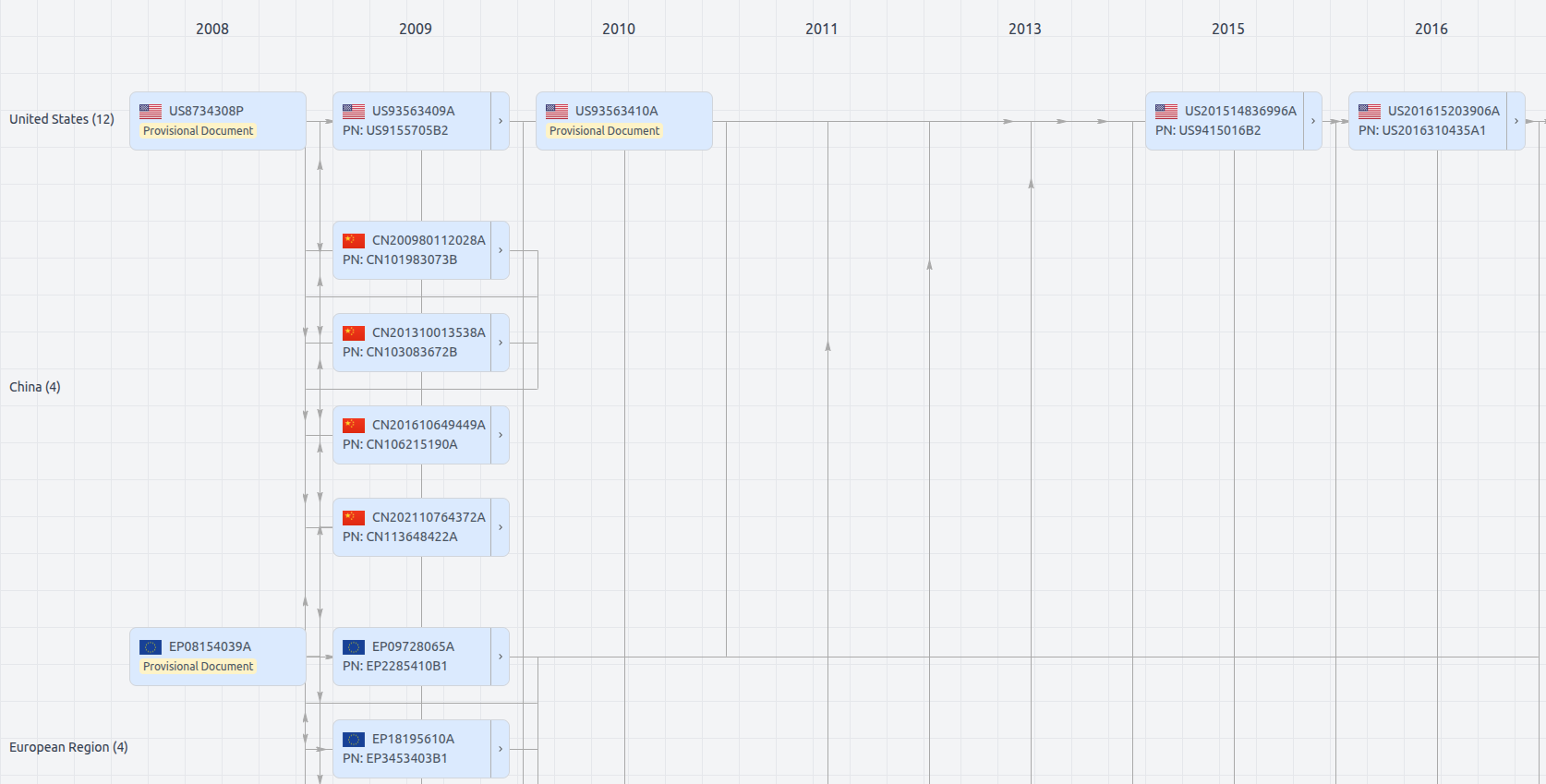
Patent Family
Patent Oppositions
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP3999180
- Application Number
- EP20753562A
- Filing Date
- Jul 16, 2020
- Status
- Granted And Under Opposition
- Apr 5, 2024
- Publication Date
- May 8, 2024
- External Links
- Slate, Register , Google Patents