Tablets Comprising Eltrombopag Olamine
Patent No. EP4218732 (titled "Tablets Comprising Eltrombopag Olamine") was filed by Novartis on Aug 1, 2007. The application was issued on Mar 26, 2025.
Patent Summary
Pharmaceutical tablet formulation for treating thrombocytopenia, comprising eltrombopag olamine, that maintains therapeutic efficacy through controlled dissolution and disintegration properties. The tablet formulation incorporates a wet granulation process using mannitol and microcrystalline cellulose as diluents, with a binder of polyvinylpyrrolidone, lubricant of magnesium stearate, and a disintegrant of sodium starch glycolate. The formulation achieves a defined particle size distribution with a specific particle size range of 10-90 microns, and a film coat containing no coordinating metals or less than 0.025 parts of Compound B.
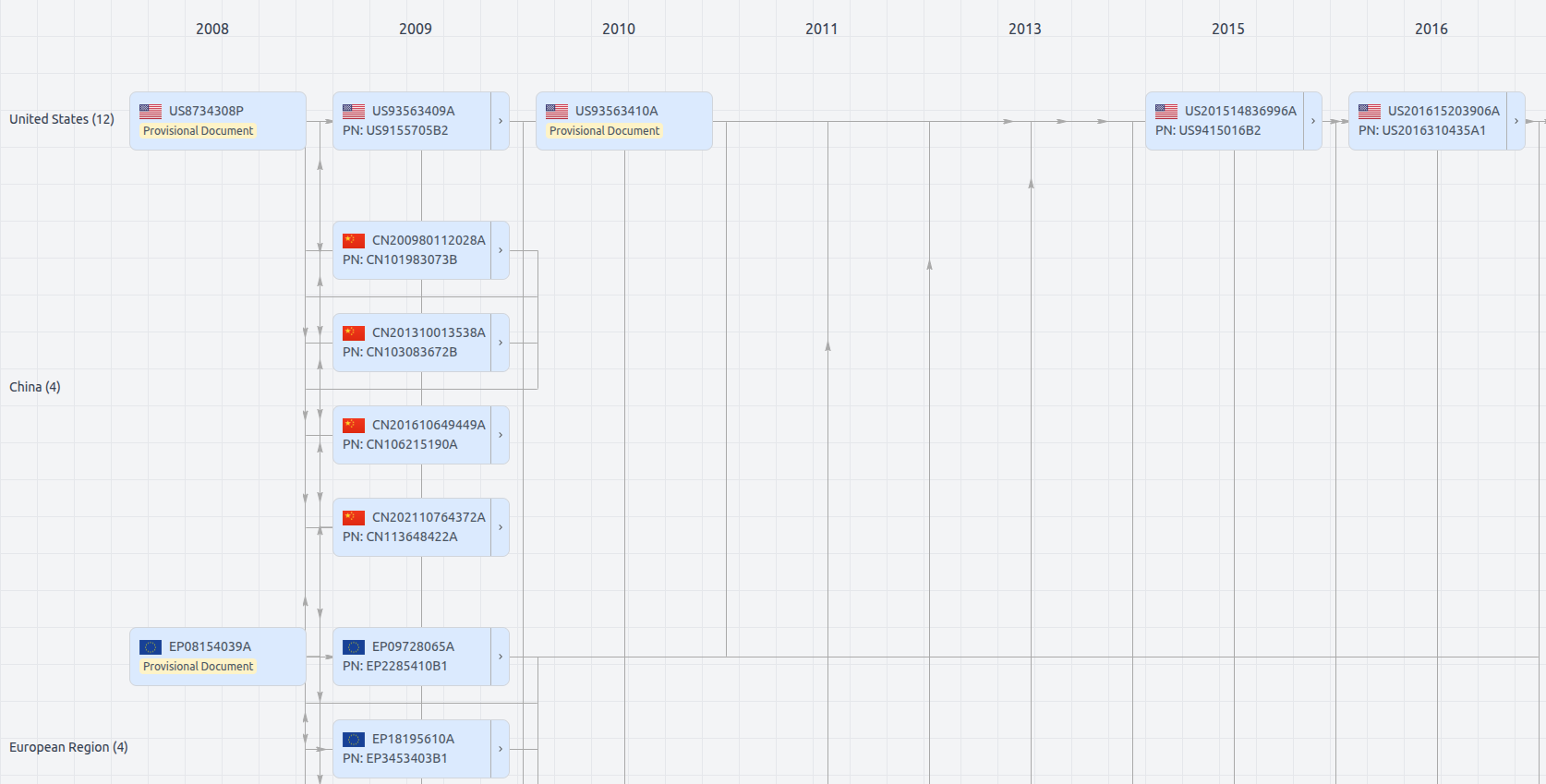
Patent Family
Patent Oppositions (3)
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
EP4218732
- Application Number
- EP23166852A
- Filing Date
- Aug 1, 2007
- Status
- Granted And Under Opposition
- Feb 21, 2025
- Publication Date
- Mar 26, 2025
- External Links
- Slate, Register , Google Patents