Novel Antitumoral Use Of Cabazitaxel
Patent No. EP2493466 (titled "Novel Antitumoral Use Of Cabazitaxel") was filed by Aventis Pharma on Oct 27, 2010. The application was issued on Mar 10, 2021.
Patent Summary
UPC Cases New
Latest UPC cases involving this patent.
| Case Number | Filing Date | Plaintiff | Proceeding Type |
|---|---|---|---|
| UPC_COA_0000027/2026 | Feb 12, 2026 | Sanofi Mature, Zentiva | Appeal |
| UPC_COA_0000026/2026 | Feb 12, 2026 | Sanofi Mature | Appeal |
| UPC_COA_0000025/2026 | Feb 12, 2026 | Sanofi, Sanofi Aventis | Appeal |
| UPC_COA_0000024/2026 | Feb 12, 2026 | Sanofi, Sanofi Aventis | Appeal |
| UPC_COA_0000023/2026 | Feb 12, 2026 | Sanofi, Sanofi Aventis | Appeal |
| UPC_COA_0000022/2026 | Feb 12, 2026 | Sanofi, Sanofi Aventis | Appeal |
| UPC_CFI_503/2024 | Sep 2, 2024 | Zentiva, Zentiva France | Counter Claim For Revocation |
| UPC_CFI_496/2024 | Sep 2, 2024 | Stada Arzneimittel, Stada Nordic | Counter Claim For Revocation |
| UPC_CFI_463/2024 | Aug 30, 2024 | Accord Healthcare, Accord Healthcare AB | Counter Claim For Revocation |
| UPC_CFI_374/2024 | Aug 29, 2024 | Betapharm Arzneimittel, DR Reddys | Counter Claim For Revocation |
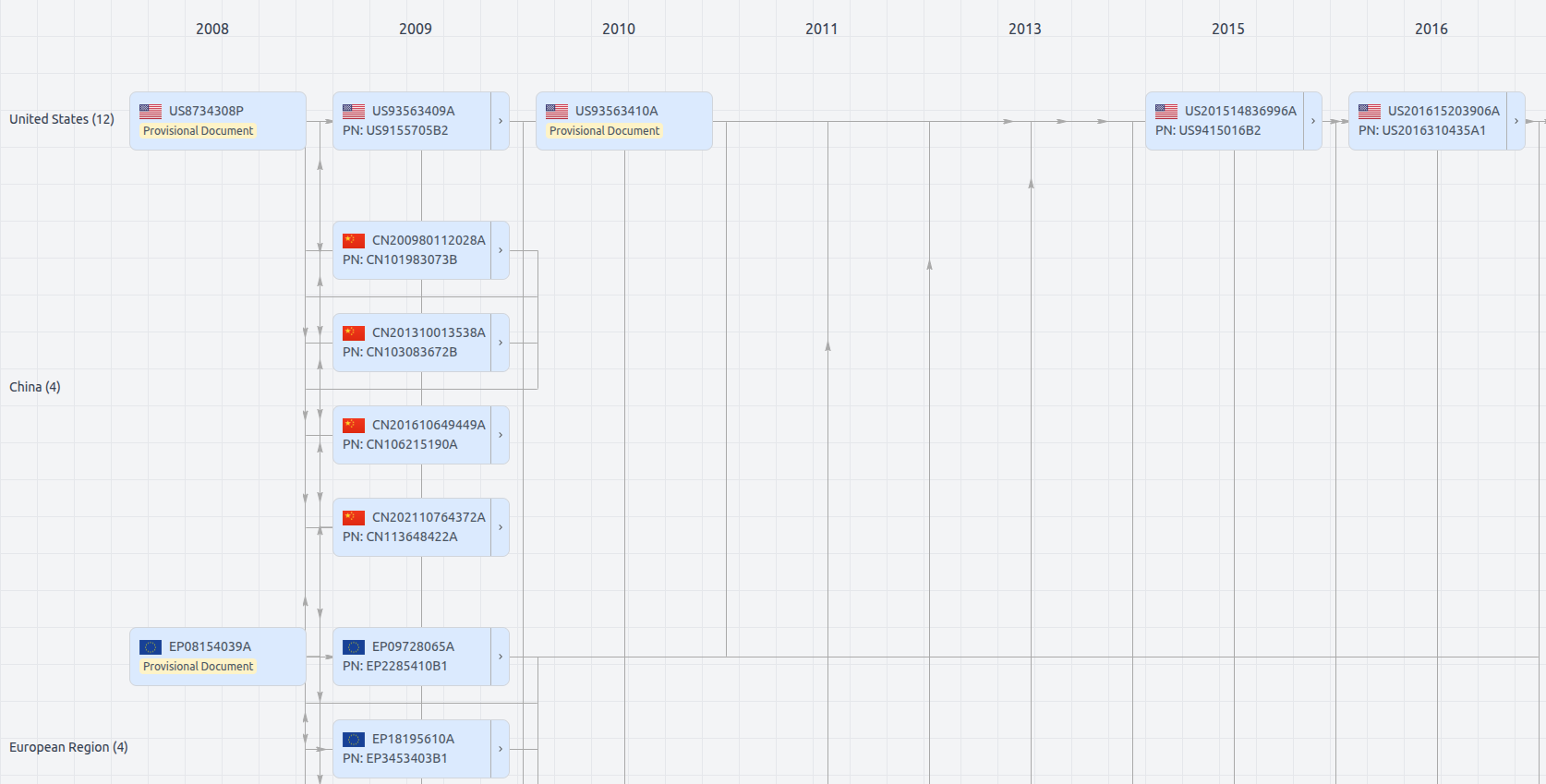
Patent Family
Patent Oppositions (15)
Patent oppositions filed by competitors challenge the validity of a granted patent. These oppositions are typically based on claims of prior art, lack of novelty, or non-obviousness. They are a key part of the process for determining a patent's strength and enforceability.
| Company | Opposition Date | Representative |
|---|---|---|
| ACCORD HEALTHCARE SLU | Aug 30, 2024 | AERA |
| ACCORD HEALTHCARE SLU | Aug 30, 2024 | BARDEHLE PAGENBERG SL |
| MAIWALD | Dec 10, 2021 | MAIWALD |
| STADA ARZNEIMITTEL | Dec 9, 2021 | HAMM & WITTKOPP |
| VOSSIUS & PARTNER PATENTANWALTE RECHTSANWALTE MBB | Dec 3, 2021 | VOSSIUS & PARTNER PATENTANWALTE RECHTSANWALTE MBB |
| GENERICS UK | Jun 8, 2021 | ELKINGTON AND FIFE |
| DR REDDYS LABORATORIES BETAPHARM ARZNEIMITTEL | Mar 31, 2021 | MAIWALD |
| FRESENIUS KABI DEUTSCHLAND | Mar 23, 2021 | FRESENIUS KABI DEUTSCHLAND |
| MEDAC GESELLSCHAFT FUR KLINISCHE SPEZIALPRAPARATE | Mar 22, 2021 | UEXKULL & STOLBERG |
| ZENTIVA KS | Mar 12, 2021 | GREINER |
| EVER VALINJECT | Mar 12, 2021 | SONN PATENTANWALTE |
| ACCORD HEALTHCARE | Mar 10, 2021 | AERA |
| ACCORD HEALTHCARE | Mar 10, 2021 | BARDEHLE PAGENBERG SL |
| GLENMARK PHARMACEUTICALS EUROPE | Mar 10, 2021 | BRAND MURRAY FULLER |
| TEVA PHARMACEUTICALS | Mar 10, 2021 | KRAUS & LEDERER PARTGMBB |
Dossier Documents
The dossier documents provide a comprehensive record of the patent's prosecution history - including filings, correspondence, and decisions made by patent offices - and are crucial for understanding the patent's legal journey and any challenges it may have faced during examination.
Date
Description
Get instant alerts for new documents
Jan 8, 2026
Jan 8, 2026
Jan 8, 2026
Jan 8, 2026
Jan 8, 2026
Jan 8, 2026
Dec 1, 2025
Dec 1, 2025
Dec 1, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 28, 2025
Nov 17, 2025
Nov 17, 2025
Nov 17, 2025
Nov 14, 2025
Nov 14, 2025
Nov 14, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 28, 2025
Oct 14, 2025
Oct 14, 2025
Oct 14, 2025
Oct 14, 2025
Sep 18, 2025
Sep 18, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 15, 2025
Sep 3, 2025
Sep 3, 2025
Aug 19, 2025
Aug 18, 2025
Jun 20, 2025
Jun 20, 2025
Jun 20, 2025
Jun 17, 2025
Jun 17, 2025
May 8, 2025
May 8, 2025
May 8, 2025
May 8, 2025
May 8, 2025
May 6, 2025
May 6, 2025
May 6, 2025
May 5, 2025
May 2, 2025
May 2, 2025
May 2, 2025
Apr 11, 2025
Apr 10, 2025
Apr 10, 2025
Apr 10, 2025
Apr 3, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Apr 1, 2025
Mar 31, 2025
Mar 31, 2025
Mar 31, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 28, 2025
Mar 21, 2025
Mar 14, 2025
Mar 14, 2025
Mar 12, 2025
Mar 12, 2025
Mar 12, 2025
Mar 11, 2025
Mar 3, 2025
Mar 3, 2025
Feb 28, 2025
Feb 28, 2025
Feb 28, 2025
Feb 28, 2025
Feb 28, 2025
Feb 28, 2025
Feb 21, 2025
Feb 20, 2025
Feb 19, 2025
Feb 19, 2025
Feb 18, 2025
Feb 17, 2025
Feb 14, 2025
Feb 14, 2025
Feb 14, 2025
Feb 14, 2025
Feb 14, 2025
Feb 14, 2025
Feb 13, 2025
Feb 11, 2025
Feb 11, 2025
Feb 11, 2025
Feb 11, 2025
Feb 10, 2025
Feb 10, 2025
Feb 10, 2025
Feb 10, 2025
Feb 6, 2025
Feb 6, 2025
Feb 6, 2025
Feb 6, 2025
Feb 5, 2025
Feb 5, 2025
Feb 5, 2025
Feb 4, 2025
Feb 3, 2025
Jan 31, 2025
Jan 31, 2025
Jan 31, 2025
Jan 30, 2025
Jan 27, 2025
Jan 27, 2025
Jan 27, 2025
Jan 27, 2025
Jan 27, 2025
Jan 27, 2025
Jan 16, 2025
Jan 14, 2025
Jan 14, 2025
Jan 14, 2025
Jan 14, 2025
Jan 14, 2025
Jan 14, 2025
Jan 10, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Jan 8, 2025
Dec 23, 2024
Dec 18, 2024
Dec 18, 2024
Dec 18, 2024
Oct 14, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 10, 2024
Oct 2, 2024
Oct 2, 2024
Oct 1, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 30, 2024
Sep 9, 2024
Sep 9, 2024
Sep 9, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Aug 30, 2024
Jul 25, 2024
Jul 15, 2024
Jul 12, 2024
Jul 12, 2024
Jul 12, 2024
Jun 18, 2024
Jun 14, 2024
Jun 14, 2024
Jun 14, 2024
Jun 14, 2024
Jun 11, 2024
Jun 11, 2024
Jun 6, 2024
Jun 4, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 31, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 27, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 24, 2024
May 23, 2024
May 23, 2024
May 23, 2024
May 22, 2024
May 22, 2024
May 22, 2024
May 21, 2024
May 21, 2024
May 21, 2024
May 21, 2024
May 21, 2024
May 21, 2024
Apr 29, 2024
Apr 29, 2024
Apr 29, 2024
Apr 29, 2024
Mar 26, 2024
Mar 22, 2024
Mar 22, 2024
Mar 22, 2024
Mar 6, 2024
Mar 6, 2024
Feb 29, 2024
Feb 28, 2024
Feb 27, 2024
Feb 27, 2024
Feb 27, 2024
Feb 27, 2024
Feb 27, 2024
Feb 27, 2024
Feb 23, 2024
Feb 23, 2024
Feb 23, 2024
Feb 23, 2024
Feb 23, 2024
Feb 23, 2024
Feb 23, 2024
Feb 22, 2024
Feb 22, 2024
Feb 22, 2024
Feb 16, 2024
Feb 15, 2024
Feb 15, 2024
Feb 15, 2024
Feb 14, 2024
Feb 12, 2024
Feb 12, 2024
Feb 12, 2024
Feb 12, 2024
Feb 7, 2024
Feb 7, 2024
Feb 7, 2024
Feb 6, 2024
Feb 2, 2024
Feb 2, 2024
Feb 2, 2024
Jan 31, 2024
Jan 29, 2024
Jan 26, 2024
Jan 26, 2024
Jan 26, 2024
Jan 26, 2024
Jan 26, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 25, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 23, 2024
Jan 15, 2024
Jan 15, 2024
Jan 15, 2024
Dec 15, 2023
Dec 12, 2023
Dec 12, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 23, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 20, 2023
Oct 19, 2023
Oct 17, 2023
Oct 17, 2023
Oct 17, 2023
Oct 17, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 13, 2023
Oct 12, 2023
Oct 12, 2023
Oct 12, 2023
Oct 12, 2023
Oct 12, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 9, 2023
Oct 5, 2023
Oct 3, 2023
Oct 3, 2023
Oct 3, 2023
Sep 26, 2023
Sep 26, 2023
Sep 26, 2023
Sep 25, 2023
Sep 25, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 21, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 13, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 8, 2023
Sep 7, 2023
Sep 7, 2023
Sep 7, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 5, 2023
Sep 4, 2023
Sep 4, 2023
Sep 4, 2023
Sep 4, 2023
Sep 4, 2023
Sep 4, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Sep 1, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 31, 2023
Aug 30, 2023
Aug 30, 2023
Aug 30, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 29, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 28, 2023
Aug 25, 2023
Aug 25, 2023
Aug 25, 2023
Aug 25, 2023
Aug 25, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 24, 2023
Aug 22, 2023
Aug 22, 2023
Aug 22, 2023
Aug 17, 2023
Aug 17, 2023
Aug 17, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 28, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 27, 2023
Jul 24, 2023
Jul 24, 2023
Jul 24, 2023
Jul 24, 2023
Jul 24, 2023
Jul 24, 2023
Jul 21, 2023
Jul 21, 2023
Jul 21, 2023
Jul 21, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 20, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 19, 2023
Jul 16, 2023
Jul 16, 2023
Jul 16, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 13, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 12, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 11, 2023
Jul 10, 2023
Jul 10, 2023
Jul 10, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 13, 2023
Mar 6, 2023
Mar 6, 2023
Mar 6, 2023
Mar 6, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 23, 2023
Jan 17, 2023
Jan 17, 2023
Jan 17, 2023
Jan 17, 2023
Jan 13, 2023
Jan 13, 2023
Dec 19, 2022
Dec 19, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Dec 7, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 17, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 16, 2022
Nov 10, 2022
Nov 10, 2022
Nov 10, 2022
Nov 8, 2022
Nov 1, 2022
Nov 1, 2022
Oct 31, 2022
Oct 31, 2022
Oct 31, 2022
Oct 31, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 26, 2022
Oct 21, 2022
Oct 21, 2022
Oct 21, 2022
Oct 15, 2022
Oct 15, 2022
Oct 15, 2022
Oct 13, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 30, 2022
Sep 27, 2022
Sep 27, 2022
Sep 27, 2022
Sep 27, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 8, 2022
Sep 2, 2022
Sep 2, 2022
Sep 2, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 10, 2022
Jun 7, 2022
Jun 7, 2022
Jun 7, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
May 6, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Apr 28, 2022
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 22, 2021
Dec 16, 2021
Dec 16, 2021
Dec 15, 2021
Dec 15, 2021
Dec 15, 2021
Dec 14, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 10, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 9, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 7, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Dec 3, 2021
Nov 24, 2021
Nov 24, 2021
Nov 24, 2021
Oct 12, 2021
Oct 12, 2021
Oct 12, 2021
Oct 8, 2021
Oct 8, 2021
Oct 8, 2021
Oct 8, 2021
Sep 20, 2021
Sep 15, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Sep 9, 2021
Aug 3, 2021
Jun 14, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
Jun 8, 2021
May 10, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
May 4, 2021
Apr 9, 2021
Apr 1, 2021
Mar 31, 2021
Mar 31, 2021
Mar 31, 2021
Mar 26, 2021
Mar 26, 2021
Mar 26, 2021
Mar 23, 2021
Mar 23, 2021
Mar 23, 2021
Mar 22, 2021
Mar 22, 2021
Mar 22, 2021
Mar 22, 2021
Mar 18, 2021
Mar 18, 2021
Mar 18, 2021
Mar 18, 2021
Mar 17, 2021
Mar 16, 2021
Mar 16, 2021
Mar 12, 2021
Mar 12, 2021
Mar 12, 2021
Mar 12, 2021
Mar 12, 2021
Mar 12, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Mar 10, 2021
Feb 15, 2021
Feb 15, 2021
Feb 15, 2021
Feb 11, 2021
Feb 11, 2021
Feb 11, 2021
Feb 11, 2021
Feb 11, 2021
Feb 11, 2021
Feb 11, 2021
Feb 10, 2021
Feb 8, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 7, 2021
Feb 4, 2021
Feb 4, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Feb 1, 2021
Jan 27, 2021
Jan 27, 2021
Jan 27, 2021
Jan 27, 2021
Jan 22, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Jan 4, 2021
Nov 30, 2020
Nov 30, 2020
Nov 30, 2020
Oct 12, 2020
Oct 12, 2020
Oct 2, 2020
Oct 2, 2020
Oct 2, 2020
Oct 2, 2020
Oct 2, 2020
Oct 2, 2020
Sep 25, 2020
Sep 25, 2020
Sep 25, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Sep 17, 2020
Aug 17, 2020
Aug 11, 2020
Aug 11, 2020
Aug 11, 2020
Aug 6, 2020
Aug 6, 2020
Aug 6, 2020
Aug 4, 2020
Aug 4, 2020
Jul 24, 2020
Jul 24, 2020
Jul 24, 2020
Jul 24, 2020
Jul 24, 2020
Jul 10, 2020
Jul 10, 2020
Jul 3, 2020
Jul 3, 2020
Jun 25, 2020
Jun 25, 2020
Jun 25, 2020
Jun 22, 2020
Jun 22, 2020
Jun 22, 2020
Jun 22, 2020
Jun 15, 2020
Jun 15, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 8, 2020
Jun 3, 2020
Jun 3, 2020
Jun 3, 2020
May 19, 2020
May 13, 2020
May 7, 2020
May 7, 2020
May 7, 2020
May 7, 2020
May 7, 2020
May 7, 2020
May 7, 2020
Apr 8, 2020
Apr 8, 2020
Apr 8, 2020
Mar 13, 2020
Mar 13, 2020
Mar 13, 2020
Mar 3, 2020
Feb 27, 2020
Feb 27, 2020
Feb 27, 2020
Dec 10, 2019
Nov 26, 2019
Nov 26, 2019
Nov 26, 2019
Nov 26, 2019
Nov 26, 2019
Oct 28, 2019
Oct 28, 2019
Jul 25, 2019
Jul 25, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jul 19, 2019
Jun 6, 2019
Jun 3, 2019
Jun 3, 2019
May 17, 2019
May 17, 2019
May 17, 2019
May 17, 2019
May 17, 2019
Mar 25, 2019
Mar 25, 2019
Mar 19, 2019
Mar 19, 2019
Mar 19, 2019
Mar 7, 2019
Mar 4, 2019
Mar 4, 2019
Mar 4, 2019
Nov 8, 2018
Nov 8, 2018
Sep 20, 2018
Sep 12, 2018
Sep 12, 2018
Sep 12, 2018
Aug 14, 2018
Aug 14, 2018
Aug 14, 2018
Aug 14, 2018
Aug 14, 2018
Aug 14, 2018
Jun 11, 2018
Jun 5, 2018
Jun 5, 2018
Jun 5, 2018
Feb 6, 2018
Feb 6, 2018
Nov 28, 2017
Nov 28, 2017
Nov 21, 2017
Nov 21, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Oct 26, 2017
Aug 2, 2017
Jul 27, 2017
Jul 27, 2017
Jul 27, 2017
Apr 20, 2017
Apr 20, 2017
Feb 9, 2017
Feb 9, 2017
Jan 31, 2017
Jan 31, 2017
Jan 31, 2017
Nov 28, 2014
Nov 28, 2014
Nov 28, 2014
Nov 28, 2014
Nov 28, 2014
Nov 28, 2014
Oct 1, 2014
Sep 24, 2014
Sep 24, 2014
Sep 24, 2014
May 26, 2014
May 26, 2014
Mar 11, 2013
Feb 26, 2013
Feb 26, 2013
Feb 22, 2013
Dec 28, 2012
Dec 28, 2012
Dec 28, 2012
Aug 8, 2012
Jun 29, 2012
May 8, 2012
May 8, 2012
Mar 19, 2012
Mar 19, 2012
Mar 19, 2012
Mar 9, 2012
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Nov 14, 2011
Oct 4, 2011
Sep 6, 2011
Sep 6, 2011
Aug 30, 2011
Aug 30, 2011
Aug 29, 2011
Aug 29, 2011
May 26, 2011
May 26, 2011
May 26, 2011
May 26, 2011
May 26, 2011
May 26, 2011
May 26, 2011
May 5, 2011
May 5, 2011
Mar 9, 2011
Mar 9, 2011
Mar 9, 2011
Jan 6, 2011
Dec 29, 2010
Nov 26, 2010
Nov 26, 2010
Nov 26, 2010
Nov 26, 2010
Nov 26, 2010
EP2493466
- Application Number
- EP10782039A
- Filing Date
- Oct 27, 2010
- Status
- Opposition Rejected
- Sep 19, 2025
- Publication Date
- Mar 10, 2021
- External Links
- Slate, Register , Google Patents